Modern large scale production of urea is based on synthesis from ammonia and carbon dioxide. This process (as originally suggested by Basaroff) was first translated into industrial scale production near 1920 by German chemists in I.G. Farben. The evolution of this original process has been driven by contributions from many countries, and its development continues around the world.
There are two main reactions involved in the synthesis of urea from carbon dioxide and ammonia; the formation of ammonium carbamate (NH2COONH4) from carbon dioxide and ammonia, and the conversion of ammonium carbamate into urea (NH2CONH2).
The reactions involved can be represented by the following equations:
NH2COONH4 (l)
<—>
NH2CONH2 (l) + H2O (l)
Overall leading to:
The formation of ammonium carbamate (reaction 1) is almost instantaneous and complete between 135 and 200 °C, provided that the pressure of the system is greater than the decomposition pressure of the ammonium carbamate at the system temperature. This formation reaction is highly exothermic; it requires continuous removal of the heat evolved. Ammonium carbamate itself is not suitable for fertilizer application because of its volatility and hygroscopic nature, and because its application leads to crop "burning".
The dehydration of carbamate into urea (reaction 2) never reaches completion. The yield of urea depends on many factors, such as the molar ratio of ammonia to carbon dioxide, the effect of water, reactor pressure, residence time, etc. Reaction 2 is an endothermic reaction; however, the quantity of heat absorbed is much smaller than the heat evolved in the formation of ammonium carbamate (reaction 1). The rate of the urea formation reaction increases rapidly above 160 °C, as can be seen in Figure 1.
The conversion of ammonium carbamate to urea does not go to completion (see Figure 1), so an additional process step is necessary for dissociation and recycle. All processes follow the same general principle: The raw materials carbon dioxide and ammonia enter the autoclave or reactor, sometimes as carbamate already, in which they (further) react and form urea. The reacted mixture then flows from the reactor into a series of dissociation and recycle process steps, operating at consequently lower pressures. In these decomposers the non-converted materials are decomposed and separated from the urea product in the solution. The urea solution is in condition to recover the final product urea. The unconverted ammonia and carbon dioxide recovered from the decomposers are typically recycled back to the reactor to reach complete conversion to urea; this is the principle of the so-called “total-recycle” process. The unconverted ammonia and carbon dioxide are typically recycled back to the reactor by dissolving them into water, forming a carbamate solution, and pumping this carbamate solution back to the reactor. In this way extra water is introduced to the reactor.
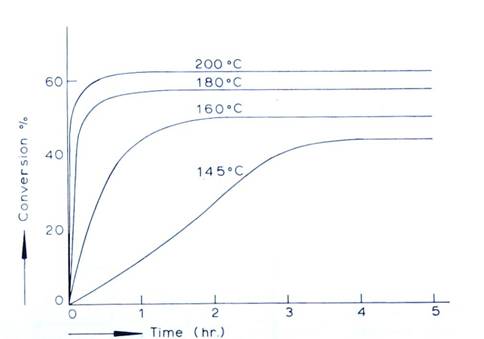
Figure 1: Conversion of ammonium carbamate to urea with time at different temperatures
A greater amount of water in the urea reactor results in a decrease of reactor conversion, as water “pushes” the chemical equilibrium of the overall reaction (reaction 3) to the left side. Figure 2 shows the influence of water (expressed as H/C ratio) on the conversion in the reactor (expressed as CO2 conversion).
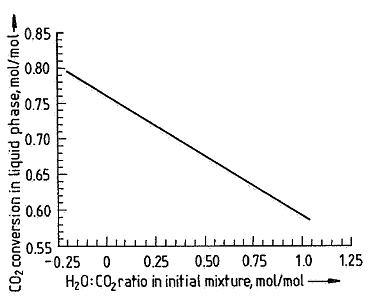
Figure 2: The influence of the H/C ratio on the CO2 conversion.
A higher H/C ratio (more water in the carbamate recycle stream) reduces the conversion in the reactor, leading to a significant decrease in efficiency of the urea process. For this reason it is critical to minimize the water content in the carbamate recycle stream of any urea plant.
The minimum water content required in the carbamate stream is determined by its crystallization point. Thus, as the carbamate solution is pumped back to the reactor, its water content must be at an optimal point. Lower water content can lead to solid formation and higher water content may prevent the urea plant from achieving maximum efficiency.
Achieving this balance is quite complex in practice. Ammonia, carbon dioxide and water can form different reaction products depending on their relative amounts, and each product has a different crystallization temperature. However, an operator’s data is typically limited to a lab analysis of the weight percentages of ammonia, carbon dioxide and water in the carbamate recycle stream. In this case the crystallization temperature level can only be estimated from the amount of water, which is obviously rather inaccurate. Furthermore, the operator needs to take care not to end up in a so-called “water recycle operation mode”, whereby the water recycle in the urea plant is continually increasing and eventually the plant load must be reduced drastically to get out of this mode.
For decades operators have had to resort to their experience in order to run this section of a urea plant smoothly. However, Virtual Materials Group and UreaKnowHow.com have recently joined forces and have been able to address the challenges involved in the prediction of the crystallization temperature of the carbamate recycle stream in a urea plant based on its composition. The results of these efforts will make an operator’s control of the water content in the carbamate recycle stream more effective, which will increase the reliability and efficiency of the urea plant.
What can an operator do to minimize the water content in the carbamate recycle stream ?
Once the crystallization temperature is known, the difference between this and the process temperature can be calculated. One obvious action that will help to control this difference is to reduce the water content. Our Carbamate Calculator can be used to calculate the influence of a water content reduction of 5 or 10% on the solution’s crystallization temperature.
Furthermore, one can vary the N/C molar ratio of the carbamate recycle stream, which will influence the process temperature. A higher process temperature allows for further reduction of the water content, but the process temperature reaches a maximum at a certain N/C ratio (azeotropic composition). Our Carbamate Calculator can be used to calculate the influence of N/C molar ratio variations of -5, -10, +5 and +10% on the crystallization temperature.
This graph is a digitalised version of Janecke phase diagrams and only the relevant composition area of carbamate recycle streams in urea plants are digitalised with high accuracy.
For most reliable temperature estimates, maintain concentrations within the following ranges:
- 25-40 wt% water and a molar N/C ratio of about 1.8-2.2
- 15-40 wt% water and a molar N/C ratio of about 2.2-2.6
These are the areas best covered by the Janecke plot and therefore the ones most accurately represented in the calculator's interpolation model.
1965 Cook CCC 3200148 Urea Process carbamate compressor
This invention by Cook and Ivo Mavrovic describes the recycle of ammonia and carbon dioxide gas mixtures by means of an adiabatic axial or centrifugal compressor. The resulting gas stream has a high temperature and is quenched by means of liquid ammonia or gaseous carbon dioxide.
1965 Mavrovic 3172911 Urea Process
This patent describes an improved recirculation process which enables a better control of the water content in the recycled carbamate. This is achieved by introducing a flash step in the first recirculation stage.
2010 09 Hanif FFC UreaKnowHow.com HP Carbamate Pumps Damage Failure Report
This case takes place in one of the three Saipem urea plants with a design capacity of 2105 mtpd. The high pressure carbamate solution recycle pumps, P102A/B, are two stage centrifugal pumps manufactured by Sundstrand, USA. The two stages of the pumps are mounted in a back arrangement on each end of the high speed shaft rotating at 16000 rpm. The low speed shaft driven by 630 KW motor rotates at 3000 rpm These pumps have a history of damage particularly at 2nd stage since commissioning in 1982 (09 damages at Pump A and 05 at Pump B). In April 2007, P102A 2nd stage was damaged after seal flush failure. After overhauling, the pump performed normal up till April 2010 when it again damaged after seal flush failure. A multi disciplinary Task Force analyzed the sequence of events and concluded that the pump thrust increased due to low seal flush and a process upset. This caused rubbing of the impeller and dynamic rotors with stators. The Bentley Nevada 72000 system could not cause tripping of the pump due to a possible deflection in the gear box frame and mounting arrangement of the probes. Several recommendations were made to improve the reliability of these pumps.
Carbamate recycle concentration
HP carbamate pumps seal water and HP NH3 pump seal oil
Impact of specific Carbamate recycle on HP loop
Check Valves in Discharge of HP Carbamate pump
Performance of low pressure carbamate condenser
Failure of High Pressure Carbamate Recycle Pump
More Information
Search for most recently updated information on UreaKnowHow.com

Virtual Materials Group Inc. is a global supplier of process simulation software for the oil, gas and chemical industries. Our suite of products includes the fully interactive steady-state and dynamic process simulator VMGSimTMand the industrially proven thermo-physical property calculation engine VMGThermoTM.
VMG's products are backed by an extremely accomplished group of scientists and engineers, dedicated to provide timely and effective world-class solutions.

UreaKnowHow.com is an independent group of urea specialists with an impressive number of years experience in designing, maintaining and operating urea plants. UreaKnowHow.com’s mission is to support, facilitate and promote the exchange of technical information within the urea industry with the target to improve the performance and safety of urea plants.
UreaKnowHow.com is the world’s largest meeting point in the urea industry with engineers & managers from over 95% of all urea plants outside China.
Visit us for more information on who we are and what we can do for you
| Ammonia [wt %] | |
| Carbon Dioxide [wt %] |
Specify concentrations within the following ranges to get reliable temperature estimates:
| Water [wt%] | N/C Ratio |
|---|---|
| 25-40 | 1.8-2.2 |
| 15-40 | 2.2-2.6 |
For more information, read About the Calculator.
| % | NH3 [wt%] | CO2 [wt%] | H2O [wt%] | N/C | Cryst T [degC] | Type |
|---|---|---|---|---|---|---|
| -10% | ||||||
| -5% | ||||||
| 0% | ||||||
| 5% | ||||||
| 10% |
| % | NH3 [wt%] | CO2 [wt%] | H2O [wt%] | N/C | Cryst T [degC] | Type |
|---|---|---|---|---|---|---|
| -10% | ||||||
| -5% | ||||||
| 0% | ||||||
| 5% | ||||||
| 10% |
